Great story by CNN reporter Brenda Goodman. Reporting is important to help protect the public from stores that sell us food when they cannot seem to be unable to do the basics.

Here is Brenda’s story:
I was doing my regular weekly grocery shopping just before Christmas when I happened to cut through the baby formula aisle to get to the dairy section at the back of the store.
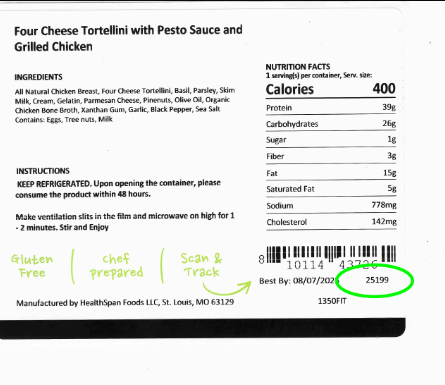
Looking up, I saw something that made me double back: at least one can of ByHeart powdered infant formula on the shelf of my local Kroger, with its recall notice from November taped underneath .
I stopped and snapped a photo with my cell phone.
I quickly sent it to my editor and several experts I work with on food safety stories, thinking I’d missed some development, but they all had the same reaction.
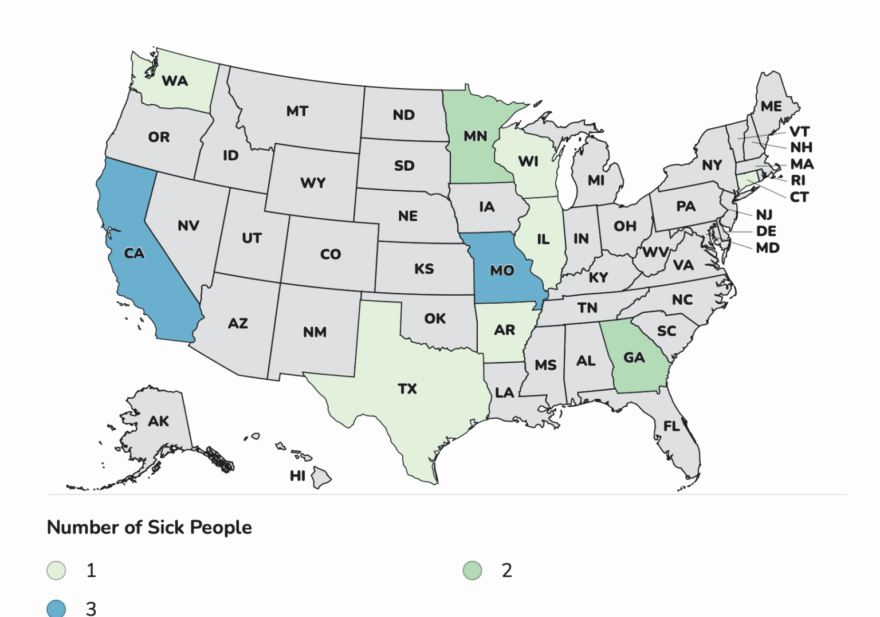
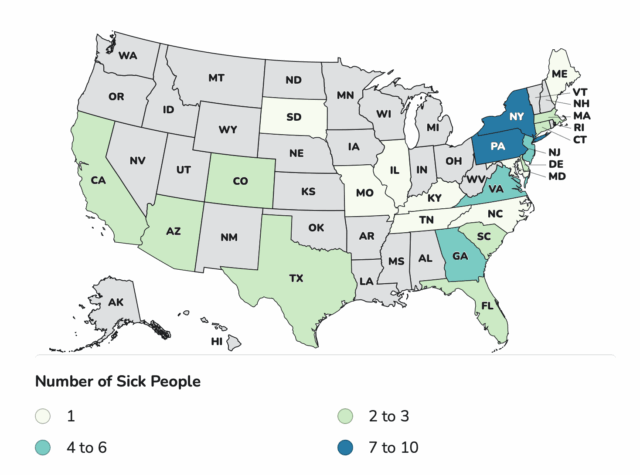
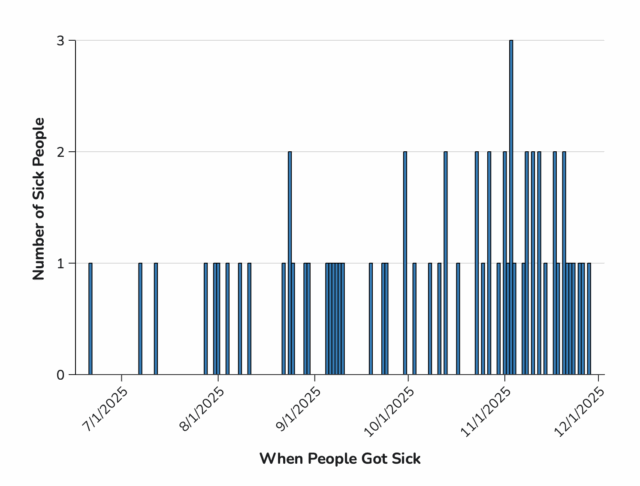
“This is nuts,” responded food safety attorney Bill Marler, who is representing several families of babies who developed infant botulism after drinking ByHeart formula. Coincidentally, that same day, he was amending the complaints he had filed to include the retailers where his clients purchased the formula, saying they hadn’t acted fast enough to get it off shelves.
Full Story – https://www.cnn.com/2025/11/25/health/infant-formula-recall-botulism-investigation