Product
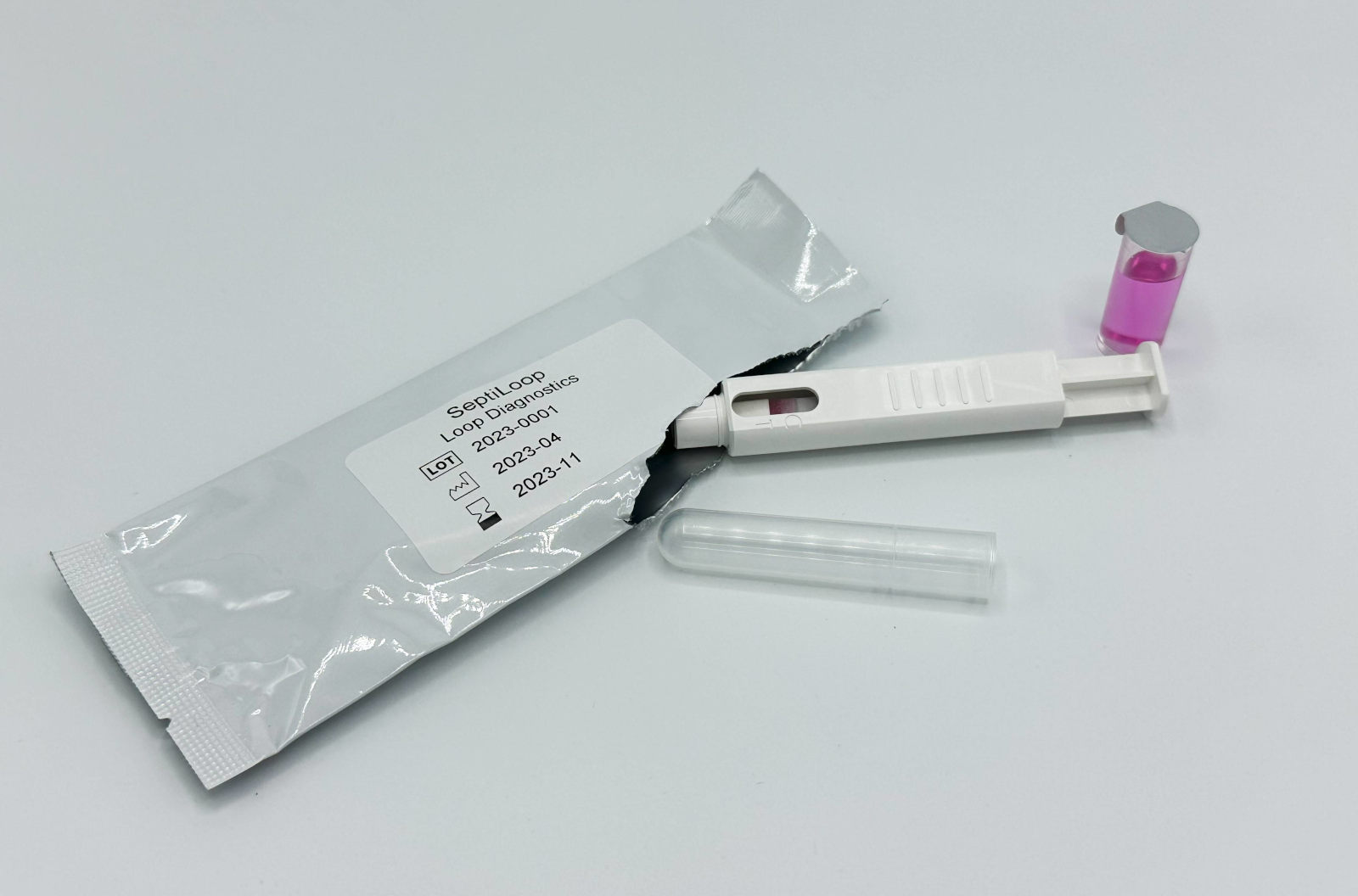
SeptiLoop® is a CE-marked (IVDR Class C) and UKCA-cleared novel in vitro diagnostic lateral flow assay that detects immunosuppression induced by bacteremia (including sepsis), by measuring the TNF-α production in LPS-stimulated whole blood.
Unlike traditional methods that focus on pathogen identification, our approach measures the status and response of the immune system of the septic patient. SeptiLoop® can detect immunosuppression induced by bacteremia as early as one hour after infection and throughout all stages of sepsis, enabling healthcare professionals an early detection and timely treatment initiation.
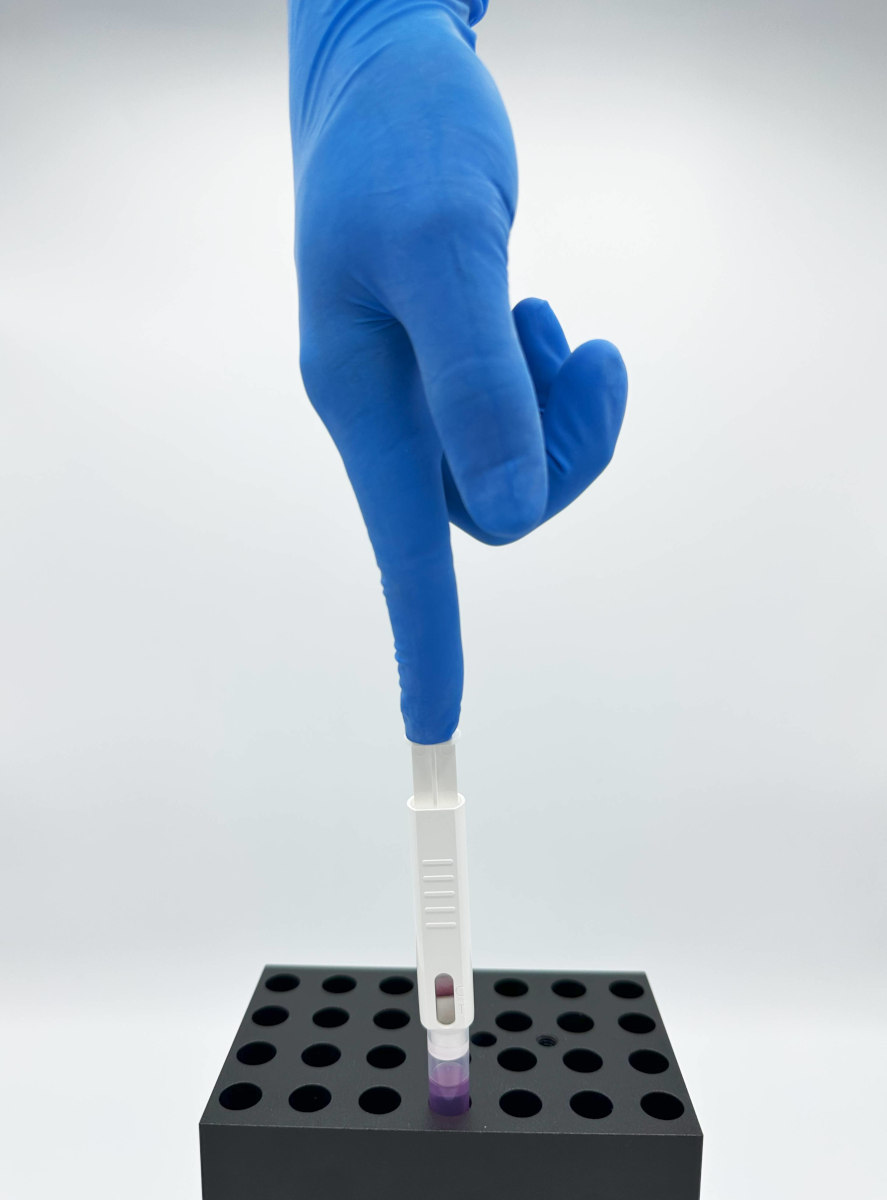
SeptiLoop® offers compelling advantages:
Early Detection
SeptiLoop’s novel approach based on immune activity allows for the detection of sepsis within the first hour of infection.
Enhanced Accuracy
SeptiLoop exhibits a remarkable 3x increase in accuracy compared to current tests
Cost-effective and User-friendly
SeptiLoop is designed as a point-of-care device, eliminating the need for extensive laboratory infrastructure.
Rapid Results
SeptiLoop provides timely results within three hours.

“This project has received funding from CDTI through the aid program for SMEs with the Horizon Europe EIC Accelerator Seal of Excellence”
ACCURATE, FAST AND COST EFFECTIVE POINT OF CARE DEVICE FOR THE EARLY DIAGNOSIS OF SEPSIS
1.797.367€
Sepsis is the leading cause of hospital deaths worldwide, with approximately 11 million deaths per year. However, there is currently no tool that provides a binary result for the diagnosis of this disease. Therefore, there is a clear need among medical professionals since current detection methods take between 6 hours and two days, rapidly decreasing the survival rate for every hour without treatment. In order to address this global clinical need, Loop DX proposes the development of a new in vitro cellular immunoassay that identifies sepsis after the first hour of bloodstream infection. In this way, SeptiLoop has been conceptualized as a Class C in vitro diagnostic device that will assist emergency physicians in diagnosing sepsis through a fast and objective technology.

“This project and business plan has received funding from CDTI through the SNEO program supported by Ministerio de Ciencia e Innovación”
Inmunoensayo de flujo lateral apoyado en un motor algorítmico para la identificación de biomarcadores específicos en el diagnóstico de sepsis.
01/02/2022 – 31/12/2022
369.200€
Our business objective is the development of a diagnostic technology for the early identification of bloodstream infections. We want to offer hospitals a device capable of performing an in vitro diagnosis (IVD) with which they can reduce mortality from this condition, patient side effects, resistance to antibiotics and the cost of managing sepsis.
The timing of diagnosis and treatments are key to controlling the disease: the sooner sepsis is confirmed, the greater the patient’s chances of survival and recovery. Current detection methods to confirm sepsis take between 6 hours and two days and it is for this reason that at Loop DX we work to reduce that detection time.
Our value chain involves designing, developing and implementing our technology to easily integrate it into hospital emergency rooms to offer healthcare professionals involved in the management of patients with sepsis a new tool to improve clinical outcomes.
eduard@loopdx.com
Lab office
Cosymbio Labs - Rent shared laboratory R&D. Carrer de Roc Boronat, 31, Sant Martí, 08005 Barcelona
Phone
+34 696 17 75 93
Follow
SeptiLoop® is the only product manufactured by Loop Diagnostics that has received CE marking under the IVDR (Class C) regulation as well as UKCA certification. All other products, packaging, and product names shown on this website correspond to prototypes displayed for illustrative purposes only. These are not available for commercialization and are intended exclusively for research and performance evaluation. SeptiLoop® IFU and/or SSP can be provided upon request by emailing enrique@loopdx.com and eduard@loopdx.com.










